We had a number of recent papers in collaboration with the department of Materials Science & Engineering (Prof. Pradeep Rohatgi’s group) on the development of new metallic materials (alloys and nano-composites) with corrosion-resistant and water-resistant properties. I think this shows how applied engineering areas, such as the metallurgy, can benefit from the fundamental research in physical chemistry and surface science.
S Behera, AP Kumar, N Dogra, M Nosonovsky*, PK Rohatgi. “Effect of microstructure on contact angle and corrosion of ductile iron: Iron-Graphite Composite” Langmuir 2019, 35, 49, 16120-16129
SK Behera, S Suri, NP Salowitz, M Nosonovsky*, PK Rohatgi “The Effect of Surface Roughness and Composition on Wetting and Corrosion of Al− Si Alloys” Israel Journal of Chemistry 2020, 60 (5-6), 577-585.
Please see also our earlier paper on how superhydrobicity can lead to corrosion-resistant materials
R Ramachandran, M Nosonovsky* Coupling of surface energy with electric potential makes superhydrophobic surfaces corrosion-resistant” Physical Chemistry Chemical Physics 17 (38), 24988-24997
_________________
* Corresponding author.
 Wetting of a metallic sample (Behera et al. Langmuir 35:16120)
Wetting of a metallic sample (Behera et al. Langmuir 35:16120)
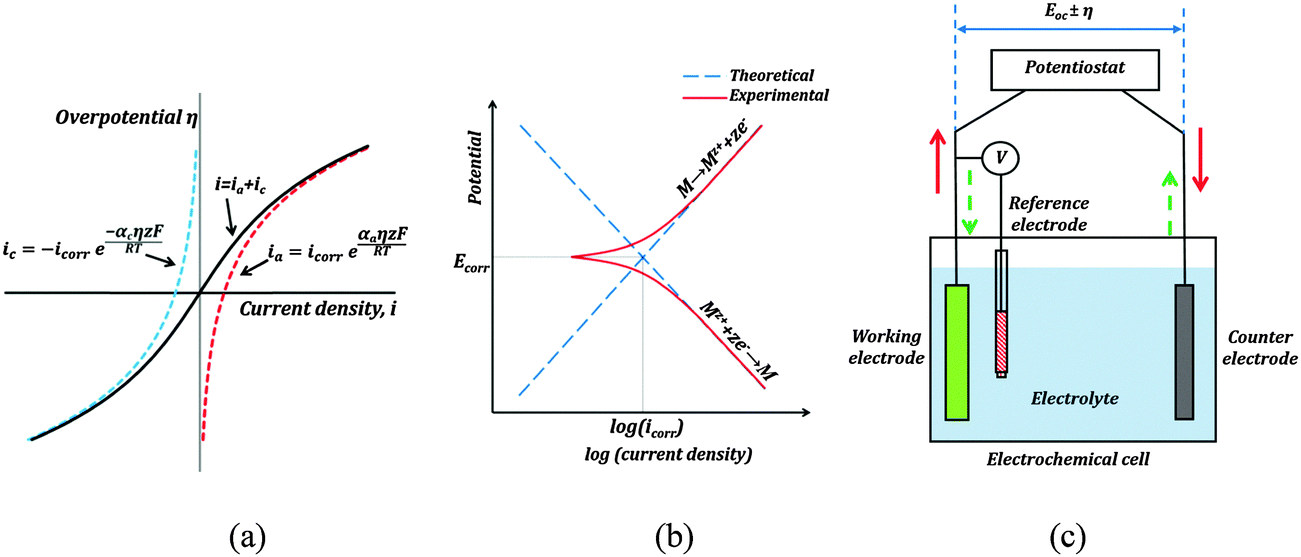 Three-electrode corrosion test (Ramachandran & Nosonovsky, PCCP 17:24988)
Three-electrode corrosion test (Ramachandran & Nosonovsky, PCCP 17:24988)
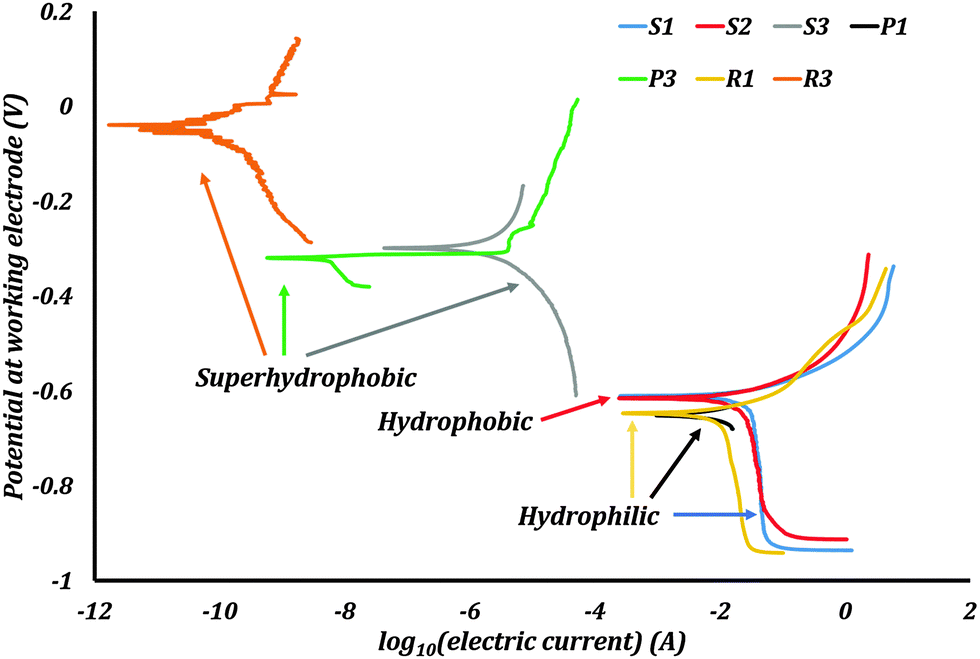 Corrosion data for hydrophilic, hydriophobic and superhydrophobic samples (Ramachandran & Nosonovsky, PCCP 17:24988)
Corrosion data for hydrophilic, hydriophobic and superhydrophobic samples (Ramachandran & Nosonovsky, PCCP 17:24988)