Current Lines of Research
Sex steroid hormones
 The primary goal of our laboratory is to pinpoint the molecules and cellular processes through which estrogens and progestins enhance memory consolidation throughout the female lifespan. We are particularly interested in how hormones affect memory in older women, because the decline of estrogens and progestins at menopause dramatically increases a woman’s risk of memory loss and Alzheimer’s disease. Because traditional hormone therapies increase risks of cancer, heart disease, and stroke, they are not recommended to treat age-related cognitive decline in women. However, the development of novel treatments that mimic the beneficial effects of estrogens and progestins in the brain, but do not produce their harmful side effects, could prevent or reverse age-related cognitive decline, thereby improving the quality of life for millions of older women. A critical step towards the development of such treatments is the discovery of the underlying molecular mechanisms in the hippocampus through which estrogens and progestins regulate memory formation (for reviews, see Frick, 2009; Frick, 2010; Frick, 2012; Frick, 2013; Fortress & Frick, 2014; Tuscher et al., 2015; Frick et al, 2015; Frick, 2015; Fortress & Frick, 2016; Frick et al., 2018a; Frick et al., 2018b; Frick & Kim, 2018; Schwabe et al., 2020; Taxier et al., 2020; Frick et al., 2020).
The primary goal of our laboratory is to pinpoint the molecules and cellular processes through which estrogens and progestins enhance memory consolidation throughout the female lifespan. We are particularly interested in how hormones affect memory in older women, because the decline of estrogens and progestins at menopause dramatically increases a woman’s risk of memory loss and Alzheimer’s disease. Because traditional hormone therapies increase risks of cancer, heart disease, and stroke, they are not recommended to treat age-related cognitive decline in women. However, the development of novel treatments that mimic the beneficial effects of estrogens and progestins in the brain, but do not produce their harmful side effects, could prevent or reverse age-related cognitive decline, thereby improving the quality of life for millions of older women. A critical step towards the development of such treatments is the discovery of the underlying molecular mechanisms in the hippocampus through which estrogens and progestins regulate memory formation (for reviews, see Frick, 2009; Frick, 2010; Frick, 2012; Frick, 2013; Fortress & Frick, 2014; Tuscher et al., 2015; Frick et al, 2015; Frick, 2015; Fortress & Frick, 2016; Frick et al., 2018a; Frick et al., 2018b; Frick & Kim, 2018; Schwabe et al., 2020; Taxier et al., 2020; Frick et al., 2020).
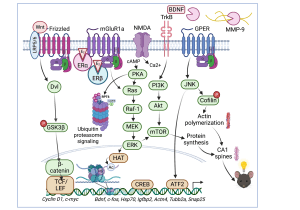
Our studies have historically focused on the hippocampus, a brain region necessary for memory formation that deteriorates in aging and Alzheimer’s disease. Our current working model of how the potent estrogen 17beta-estradiol (E2) enhances memory in the dorsal hippocampus is illustrated to the left (Frick, 2015). We have discovered that several membrane-associated receptors are necessary for E2 to enhance object recognition and spatial memory, including ERalpha, ERbeta, metabotropic glutamate receptors, and NMDA receptors (Boulware et al., 2013; Kim and Frick, 2017 Lewis et al., 2008). Activation of these receptors in the dorsal hippocampus triggers the rapid activation of numerous cell-signaling pathways, including ERK and mTOR. This activation is necessary for E2 to enhance hippocampal memory in young and middle-aged female mice (Fernandez et al., 2008; Fan et al., 2010; Lewis et al., 2008; Fortress et al., 2013b). In collaborative work with Drs. Vicky Luine (Hunter College) and Maya Frankfurt (Hofstra North Shore-LIJ School of Medicine),  we also found that dorsal hippocampal ERK and mTOR activation are necessary for E2 to increase dendritic spine density in both the dorsal hippocampus and medial prefrontal cortex of young female mice (Tuscher et al., 2016a). Interestingly, activation of the membrane estrogen receptor GPER enhances hippocampal memory formation via JNK signaling, rather than ERK signaling (Kim et al., 2016), suggesting that GPER may not function as an estrogen receptor in the hippocampus. In support, our subsequent work showed that E2’s ability to increase CA1 dendritic spine density does not depend on GPER activation. Rather, GPER’s ability to increase CA1 spine density and enhance memory are dependent on JNK signaling and actin polymerization (Kim et al., 2019). Collectively, these findings suggest that ERalpha, ERbeta, and GPER rapidly active cell signaling in the dorsal hippocampus to promote spine plasticity and memory consolidation, but appear to do so via different signaling mechanisms.
we also found that dorsal hippocampal ERK and mTOR activation are necessary for E2 to increase dendritic spine density in both the dorsal hippocampus and medial prefrontal cortex of young female mice (Tuscher et al., 2016a). Interestingly, activation of the membrane estrogen receptor GPER enhances hippocampal memory formation via JNK signaling, rather than ERK signaling (Kim et al., 2016), suggesting that GPER may not function as an estrogen receptor in the hippocampus. In support, our subsequent work showed that E2’s ability to increase CA1 dendritic spine density does not depend on GPER activation. Rather, GPER’s ability to increase CA1 spine density and enhance memory are dependent on JNK signaling and actin polymerization (Kim et al., 2019). Collectively, these findings suggest that ERalpha, ERbeta, and GPER rapidly active cell signaling in the dorsal hippocampus to promote spine plasticity and memory consolidation, but appear to do so via different signaling mechanisms.
Our findings that canonical Wnt signaling is necessary for memory consolidation (Fortress et al., 2013a) raise the possibility that Wnt signaling may also play a role in E2-induced memory enhancement. These findings form the basis of our R01 grant from the National Institute of Mental Health to study the roles of canonical Wnt signaling and the neurotrophin BDNF in mediating the effects of E2 on memory consolidation in female and male mice. One recent study from this project showed that the endogenous Wnt signaling inhibitor Dkk-1 prevents E2 from enhancing memory, but perhaps via non-canonical Wnt signaling (Taxier et al., 2019). Most recently, we demonstrated that the high-affinity BDNF receptor TrkB is essential for E2 to enhance object recognition and spatial memory consolidation (Gross et al., 2021), as is matrix metalloproteinase 9 (MMP9), an extracellular protein that cleaves pro-BDNF into BDNF in the extracellular space (Gross et al., 2022).
 Downstream from cell signaling, we have found that the ability of E2 to enhance memory in young and middle-aged females depends on rapid activation of the epigenetic processes histone acetylation and DNA methylation (Zhao et al., 2010; Zhao et al., 2012; Fortress et al., 2014). Other work from the lab has identified genes in the dorsal hippocampus whose expression is regulated by E2 (Pechenino & Frick, 2009). We have also used chromatin immunoprecipitation (ChIP) to show that E2 increases acetylation of promoters of the BDNF gene (Fortress et al., 2014). More recently, we showed that activation of the high-affinity BDNF receptor, TrkB, is essential for E2 to enhance memory consolidation in female mice (Gross et al., 2021). In a related project, we collaborated with Drs. Mahmun Hossain, David Frick, and Douglas Steeber of UWM’s Chemistry and Biological Sciences departments to study the effects on memory of selective histone deacetylase inhibitors. Our findings show that a novel HDACi can enhance memory consolidation in male mice (Belayet et al., 2022).
Downstream from cell signaling, we have found that the ability of E2 to enhance memory in young and middle-aged females depends on rapid activation of the epigenetic processes histone acetylation and DNA methylation (Zhao et al., 2010; Zhao et al., 2012; Fortress et al., 2014). Other work from the lab has identified genes in the dorsal hippocampus whose expression is regulated by E2 (Pechenino & Frick, 2009). We have also used chromatin immunoprecipitation (ChIP) to show that E2 increases acetylation of promoters of the BDNF gene (Fortress et al., 2014). More recently, we showed that activation of the high-affinity BDNF receptor, TrkB, is essential for E2 to enhance memory consolidation in female mice (Gross et al., 2021). In a related project, we collaborated with Drs. Mahmun Hossain, David Frick, and Douglas Steeber of UWM’s Chemistry and Biological Sciences departments to study the effects on memory of selective histone deacetylase inhibitors. Our findings show that a novel HDACi can enhance memory consolidation in male mice (Belayet et al., 2022).
Our studies have recently grown to encompass the medial prefrontal cortex (mPFC), which we have shown using DREADDs is necessary for object recognition and spatial memory consolidation (Tuscher et al., 2018). E2 infused into the mPFC enhances the consolidation of both types of memory, and studies using DREADD-mediated inactivation of the mPFC have demonstrated that both the mPFC and dorsal hippocampus must be activated concurrently for E2 to facilitate memory consolidation (Tuscher et al., 2019). The nucleus reuniens of the thalamus (RE) has been hypothesized to mediate communications between the dorsal hippocampus and mPFC, so we have also explored a role for this brain region in memory consolidation. We recently showed that DREADD-mediated inactivation of the RE blocks spatial memory consolidation and reduces neural activity (Schwabe et al., 2021), so future work will examine how this nucleus might contribute to estrogen-mediated hippocampal-mPFC interactions.
We also maintain several E2-related active collaborations with faculty at UWM and elsewhere on topics including:
- Novel ERbeta development: We have collaborated with Drs. Daniel Sem (Concordia University Wisconsin) and William Donaldson (Marquette University) to develop new estrogen receptor beta agonists for reducing the symptoms of menopause including memory loss, hot flashes, depression, and anxiety. Acute treatment with our lead compound, EGX358, enhances memory consolidation via 3 routes of administration, including oral (Hanson et al., 2018). More recently, we found that long-term (2-3 months) oral administration not only enhances memory consolidation but also reduces hot flashes in ovariectomized mice (Fleischer et al., 2021). As such, this compound may be a promising therapeutic for menopausal women. This work has been or is currently supported by grants from the National Institute of General Medical Sciences, National Institute on Aging, the Alzheimer’s Association, the UW System, and the UWM Research Foundation, and is the foundation of our company, Estrigenix Therapeutics, Inc.
- Sex, E2, ERbeta, and APOE genotype in Alzheimer’s: As part of a collaboration with Drs. Mary Jo LaDu and Leon Tai (University of Illinois at Chicago), we have received multiple grants from the Alzheimer’s Association to study the effect of apolipoprotein E genotype, sex, E2 treatment, and ERbeta agonist treatment on memory in mouse model of Alzheimer’s disease. Our recent papers support a detrimental effect of APOE4 genotype on memory, anxiety-like behaviors, CA1 and mPFC dendritic spine density, and that two copies of the APOE4 allele blocks the beneficial effects of E2 on memory and CA1 spine density (Taxier et al., 2022a; Taxier et al., 2022b; Taxier et al., 2022c). In our current Alzheimer’s Association grant, we are testing the extent to which a novel highly selective ERbeta agonist regulates memory, synaptic plasticity, and AD pathology in APOE3/3, APOE3/4, and APOE4/4 female mice.
- Protein degradation: Through our new R01 grant, we will collaborate with Dr. Tim Jarome of Virginia Tech to study the role protein degradation in E2-induced memory and dendritic spinogenesis, as well as use mass spectroscopy to identify specific proteins targeted by E2.
- Estrogenic engrams: We are working with Dr. Steve Ramirez of Boston University to use his viral Tet-Tagging approach to discover the identity hippocampal cells are recruiting into an episodic engram by learning and E2, and determine if these cells are necessary for learning.
- Gene expression: Together with Dr. Janine Kwapis of Penn State, we are using RNAseq to study effects of E2 and learning on hippocampal gene expression in mice of both sexes.
- Locally-synthesized E2: In collaboration with Dr. Luke Remage-Healey (U Mass Amherst), we first examined the role of E2 synthesized within the dorsal hippocampus in memory formation. Our data indicate that hippocampally-synthesized E2 is necessary for the formation of object recognition and spatial memories in ovariectomized female mice (Tuscher et al., 2016b) and gonadectomized male mice (Koss et al., 2019). This work suggests that object learning triggers hippocampal E2 synthesis in both sexes. With Dr. Polymnia Georgiou at UWM, we are currently testing this hypothesis and examining roles for local E2 synthesis in the prefrontal cortex in memory consolidation.
- E2 and addiction: In collaborations with Drs. Devin Mueller (Kent State University) and John Mantsch (Marquette), we have shown in female rats that E2 is necessary for the extinction of a cocaine-induced place preference (Twining et al., 2013), possibly via mediating intrinsic excitability in the infralimbic mPFC (Yousuf et al., 2019), and that E2 plays a key role in potentiating reinstatement of cocaine seeking (Doncheck et al., 2018).
Finally, although much of our work has focused on E2, a similar line of research demonstrates that progesterone can also rapidly enhance memory consolidation in female mice throughout adulthood (e.g., Harburger et al., 2008, 2009; Lewis et al., 2008; Orr et al., 2009), and do so rapidly via ERK and mTOR activation in the dorsal hippocampus (Orr et al., 2012). Our most recent findings demonstrate that these effects are mediated via membrane progesterone receptors, whereas intracellular progesterone receptors appear to regulate memory consolidation by activating canonical Wnt signaling (Fortress et al., 2015).
Sex differences
Our early work on sex differences in memory highlighted the existence of sex differences in age-related memory and neurochemical decline in mice (e.g., Frick et al., 2000; Frick et al., 2002) and demonstrated that young male mice outperform young female mice in object recognition (Frick & Gresack, 2003) and working memory (Gresack & Frick, 2003) tasks. Interestingly, our work with human subjects shows a different pattern of sex differences. In Yale undergraduates, we found that spatial memory tested in a virtual radial arm maze does not differ between men and women, whereas object memory is superior in women relative to men (Levy et al., 2005). Whether a sex differences in observed in a particular test of learning and memory is influenced by numerous factors, including experimental methodology and stress. This latter point is highlighted in our recent study showing that prenatal stress impairs spatial memory in both adult male and female mice, but induces greater epigenetic and corticosterone alterations in the hippocampus of females (Benoit et al., 2015). We discuss these issues in a recent review paper published as part of a special issue on sex differences (Koss & Frick, 2017).
We are also beginning to examine the molecular bases of sex differences in memory. In one previous study, we found that a sex difference (favoring males) in contextual fear conditioning is associated with increased activation of ERK in the ventral, but not dorsal, hippocampus in males relative to females (Gresack et al., 2009). More recently, we showed that dorsal hippocampal infusion of E2 enhances hippocampal memory consolidation similarly in male and female mice, but not via ERK activation as in females (Koss et al., 2018). Instead, the ability of E2 to enhance memory in males may be due to ERK-independent CREB phosphorylation (Koss et al., 2018).
In another recent study examining effects of aromatase inhibition on memory, we found that hippocampal E2 is necessary for memory consolidation in gonadectomized male mice as in ovariectomized females (Koss et al., 2019). However, this dependence on hippocampal E2 was not found in gonadally-intact males (Koss et al., 2019), suggesting that activation of androgen receptors by gonadally-synthesized androgens protects against memory impairments caused by aromatase inhibition.
Finally, we have also started examining sex differences in how learning influences protein degradation. This work focuses on the ubiquitin proteasome system, or UPS, which is a primary method of protein disposal on which learning and memory depend. We found that object learning in male and female mice increases dorsal hippocampal UPS activity more in the synaptic fractions from males and cytosolic fractions from females (Beamish et al., 2022). Learning also activated the UPS more in the dorsal hippocampus than in the medial prefrontal cortex.
Environmental enrichment
 Finally, we also have a long-standing interest in studying how various types of environmental stimulation affect the response to aging, hormone treatment, and stress. We have found that environmental enrichment, consisting of both cognitive stimulation and physical exercise, can improve various types of hippocampal-dependent memory (e.g., spatial and object memory) and enhance hippocampal function (e.g., synaptic protein levels, growth factor levels) in young, middle-aged, and aged male and female mice (e.g., Frick et al., 2003; Frick & Fernandez, 2003; Lambert et al., 2005; Bennett et al., 2006). These studies demonstrate somewhat different effects of enrichment in males and females, with respect to both memory and synaptic protein levels (Harburger et al., 2007a). We have also tried to determine which aspects of the enriched environment are particularly important for cognition enhancement. Our data, thus far, indicate that exercise most effectively improves spatial memory in young and middle-aged females, whereas both exercise and cognitive stimulation are effective for aged females (Lambert et al., 2005; Harburger et al., 2007b). Finally, we have also shown that enrichment reduces the ability of E2 to enhance memory in young and middle-aged female mice (Gresack & Frick, 2004; Gresack et al., 2007).
Finally, we also have a long-standing interest in studying how various types of environmental stimulation affect the response to aging, hormone treatment, and stress. We have found that environmental enrichment, consisting of both cognitive stimulation and physical exercise, can improve various types of hippocampal-dependent memory (e.g., spatial and object memory) and enhance hippocampal function (e.g., synaptic protein levels, growth factor levels) in young, middle-aged, and aged male and female mice (e.g., Frick et al., 2003; Frick & Fernandez, 2003; Lambert et al., 2005; Bennett et al., 2006). These studies demonstrate somewhat different effects of enrichment in males and females, with respect to both memory and synaptic protein levels (Harburger et al., 2007a). We have also tried to determine which aspects of the enriched environment are particularly important for cognition enhancement. Our data, thus far, indicate that exercise most effectively improves spatial memory in young and middle-aged females, whereas both exercise and cognitive stimulation are effective for aged females (Lambert et al., 2005; Harburger et al., 2007b). Finally, we have also shown that enrichment reduces the ability of E2 to enhance memory in young and middle-aged female mice (Gresack & Frick, 2004; Gresack et al., 2007).