Macromolecular Crystallography
We are using macromolecular crystallography to characterize the static structures of heme and non-heme iron proteins and other biochemically, biologically and pharmaceutically interesting proteins. Pure protein samples and crystals are prepared in our wet lab and examined either in-house on our Xenocs microfocus X-ray source or at a synchrotron beamline. We are using mainly the BioCARS facility at the Advanced Photon Source in Argonne near Chicago. The image below shows a gallery of selected structures from the Schmidt lab. The common denominator in all these structures is the existence of a central chromphore that is essential for the function of the protein. Details of this function are investigated further by Time-Resolved Crystallography.
Single Particle Cryo-Electronmicroscopy
Single particle cryo-EM is complementary to X-ray crystallography since it allows for full relaxations of biological macro-molecules to occur as well as for the possibility to determine near atomic resolution structures of specimen stubbornly resisting crystallization. The former is important when macromolecular reactions are investigated, the latter is caused for example by unstable protein specimen that decay before they form a crystal. We encountered both challenges when working on bacteriophytochromes (BphPs) that show large relaxations after the absorption of red or far-red light. Furthermore, a typical BphP has histidine kinase (HK) activity as part of a two-component signaling pathway in bacteria. The HK domain is rather flexible and labile. It separates from the remainder of the BphP within a few days even when kept at lower temperatures in the fridge. Since 20 years, crystallization of a full-length BphP with HK activity was attempted without success. However, the remaining BphP without the HK forms formidable crystals that diffract to highest resolution. A combination of crystallography and cryo-EM is capable to structurally characterize the complete photocycle after light absorption. Below we show three cryo-EM densities of intact myxobacterial BphP. Important is panel (b) that displays the structure of a BphP heterodimer with one subunit in the red absorbing Pr state and the other in the far-red absorbing Pfr state. We were the first who ever attempted and succeeded to solve a biological macromolecular structure ab-initio with single particle cryo-EM at the University of Wisconsin-Milwaukee. Results are published and can be found here: https://www.science.org/doi/10.1126/sciadv.adq0653. In addition, there is great expertise in our department (group of Prof. Peter Schwander) to detect structural heterogeneity in cryo-EM data using manifold-EM and other methods. It is our goal to implement time-resolution into our investigations of the BphP with cryo-EM. This will reveal an intimate insight into light perception and signal transduction by bacterial phytochromes.
Time-Resolved Macromolecular Crystallography (The 4th Dimension)
Time-Resolved crystallography is a unique method to examine both structure and kinetics at the same time. Structure can be probed with atomic resolution and kinetics with 100 picosecond time resolution. This way, time-resolved crystallography genuinely unifies structure with kinetics. Time-resolved crystallography was pioneered by Keith Moffat and collegues in the late 80th and 90th of the previous century and became mature in the new millenium. Since the X-ray data must be collected ultrafast within 100 ps, the conventional rotation method had to be given up in favour of the Laue method. It required immense pioneering effort to cope with the complexity of the polychromatic data. There exists a single beamline in the U.S. where these types of experiments can be performed, BioCARS 14-IDB. This beamline has been upgraded recently and features now a tighly focused polychromatic X-ray beam in conjunction with a laser facility consisting of picosecond and nanosecond lasers. Single 100 ps X-ray pulse exposures produce analyzable diffraction pattern. That paves the way to investigate non-reversible (non-cyclic) reactions that commonly occur in enzymes and other proteins.
The heart of BioCARS 14-IDB, Advanced Photon Source
Five-Dimensional Crystallography (The Twilight Zone)
When the temperature is increased, molecular processes speed up. The temperature is a great controllable variable to extract new properties of biological macromolecules. You find an introduction here: Up-up and away to the twilight zone!
Serial Femtosecond Crystallography, Time-Resolved Serial Femtosecond Crystallography
Macromolecular crystallography as it exists nowadays might fade into oblivion with the advent of the brightest X-ray sources the world has ever seen, the free electron lasers for hard X-rays (X-ray FELs). One bottleneck of crystallography is to grow crystals large enough that diffraction patterns can be collected from them with existing X-ray sources. It is realtively easy to grow nano and microcrystals, but it may take years to optimize conditions to grow large single crystals especially from membrane proteins. With crystals becoming smaller and smaller a limit is reached beyond which the crystals cannot be made smaller without destroying them by the amount of X-rays required to collect even single diffraction pattern. Although damage by the depostited X-ray dose is largely suppressed by keeping the crystals cold at cryogenic temperatures around 100 K, this limit is around 2 to 5 micrometers. Beyond that it is very difficult to collect damage-free diffraction patterns. Then came the X-ray FELs. They provide femtosecond X-ray pulses with a couple of trillions (1012) quasi-monochromatic X-ray photons in a single 40 femtosecond pulse. For comparison, the strongest 3rd generation synchrotron provides on the order 50 billion (5×1010) polychromatic X-ray photons in a 100 picosecond pulse. The temporal photon density (number of X-ray photons per unit time) at the X-ray FEL is about 50,000 times larger than at the synchrotron. This ratio is even more crazy for those quasi-monochromatic photons, say within a 0.1 % bandwidth: here, the X-ray FEL provides even 50 million times more quasi-monochromatic photons per unit time than the synchrotron. In addition, this large flux can be focused to an extremly small focal spot, since as the name suggests the X-ray FEL is a laser, which features a coherent beam with very small divergence or crossfire. It is this small crossfire that is mainly responsible for the immense increase in brilliance which is 9 to 10 orders of magnitude larger for the X-ray FEL compared to the synchrotron. Focal spots as small as 100 nm are already reality at the Linac Coherent Light Source (LCLS). This provides the means necessary to interrogate nanocrystals. However, when this huge number of X-ray photons are incident on these very small crystals, they explode … “bam”, because the damage they suffer is several orders of magnitude larger than at the safe-dose at which the damage can be tolerated. Amazingly, a diffraction pattern is collected before the explosion. This is called the “diffraction-before-destruction” principle. Fantastic!!! The crystal size limit has been overcome. There is no need to grow large crystals anymore. Crystals with only a few hundred unit-cells can be investigated easily. Of course, since they are destroyed, the method requires a constant stream of fresh tiny crystals that are intercepted in random orientation by the X-ray FEL beam. Since a serial stream of crystals is involved, the X-ray FEL pulses are femtosecond long, and the crystals are nanosized, this method has been names Serial Femtosecond Nano-Crystallography (SFX). Special injectors had to be developed to provide the stream of crystals. There exist a number of different flavors of injectors to date. Some consume a lot of protein and are more easy to handle, some do not, but they require additional techniques. In addition, since the diffraction patterns are obtained from tiny crystals in random orientation, each and every diffraction pattern (snaphot) that contains Bragg spots must be indexed anew. Since the X-ray FEL beam is quasi-monochromatic, very partial reflection intensities are obtained from each snapshot. In order to reconstruct integrated reflection intensities, a large number of these partial observations must be averaged for each reflection. For a good dataset, each reflection is observed as much as 1000 times in as much as 100000 snapshots that could be indexed successfully out of 2 million diffraction patterns collected. Just for comparison, here are the numbers from conventional crystallography: (i) only one diffraction pattern needs to be indexed, (ii) each reflection is observed on the order of 10 times on subsequent diffraction patterns which differ by a rotation of 0.1 deg, hence the rocking curve is faithfully traced for each reflection, (iii) the number of diffraction pattern is typically smaller than 1000. To make things more complicated for the X-ray FELs, the first existing light source that provides this required magnitude of flux, the LCLS, is relatively slow, because its pulse repetition rate is only 120 Hz. With a liquid jet injector only 1 of 100,000 crystals (0.001%) will ever be interrogated by the LCLS X-ray beam, the rest (99.999%) will be dumped as waste. Fortunately, the Europan XFEL (EXFEL) became online in 2016 and features a repetition rate (up to 4 MHz) which is several orders of magnitude higher than that at the LCLS. Rather than consuming grams of protein at the LCLS, we will be using only milligrams at the EXFEL. It takes a shift to collect a dataset at the LCLS today, and it will take only a few minutes at the EXFEL. It has been established that the maximum X-ray pulse repetition rate is too fast for the existing injectors, and a previous FEL X-ray pulse will disturb the jet so that the next pulse is missing the jet. The repetition rate of the X-ray pulses must be reduced to suitable values, for example to 500 kHz, that are commensurate with the jet speeds of the injectors.
Interestingly, SFX can be also operated in a time-resolved fashion (TR-SFX). Then, a reaction in the crystals has to be initiated upstream, before the crystals are interrogated (and destroyed) by the X-ray FEL beam. The easiest way to initiate a reaction is with a pulsed optical laser, whose pulses must be synchronized to the FEL X-ray pulses. Yes, this works! However, most biological reactions cannot be initiated by light. Other methods need to be developed. The amazing thing with SFX is that the crystals are so small. When crystals are so small, diffusion times are also small (hence fast). Reactions can be swiftly initiated by diffusion. It takes microseconds for the substrate to diffuse into the nanocrystals as compared to seconds or minutes for macroscopic crystals. The ease and simplicity of diffusion may revolutionize time-resolved structural investigations in a sense that now many biologically, pharmaceutically and medically important enzymes can be observed in action. Our group in collaboration of a large international team of researchers from the United States, England and Germany were the first who did a successful experiment at near atomic resolution using time-resolved serial femtosecond crystallography (TR-SFX) at the beamline CXI at the Linac Coherent Light Source. We used really small crystals of photoactive yellow protein and injected them into the X-ray FEL beam. A short time interval earlier, a reaction had been started in the crystals by a nanosecond laser. We were able to observe the structural changes at time points of 1 us and 10 ns after laser initiation at a spatial resolution of 1.6 A. Not only was this the first successful TR-SFX experiment but we also hold the record for the highest spatial resolution achieved at the X-ray FEL for protein crystals sofar. We are looking forward when this record will be broken. We all suspect that we can do it, and it will not take long. The succesfull TR-SFX experiment has been published on Dec. 5 2014 in Science.
Structures determined from comprehensive time-series collected at the synchtrotron (BioCARS) and from the X-ray FEL (CXI) were used to produce a movie of the photocycle (please be patient, movie is kind of big). Please feel free to use the movie, but cite this web-page!
Other Techniques
Moessbauer Spectroscopy
To investigate the dynamics of biological macromolecules we are using energy resolution rather than time-resolution. Usually, X-rays (as is the case for all photons) come in packages of a certain lifetime which is, for conventional sources, typically in the femtosecond time-regime. If these photons are scattered (or absorbed) all processes faster than that characteristic lifetime can be observed. In biological macromolecules processes faster than femtoseconds are rare. This means, that conventional X-rays generate snaphots of, on that timescale, frozen molecules. However, there are X-ray sources that generate packages with lifetimes of several hundreds of nanoseconds. These X-rays originate from the nucleus of certain elements and, hence, they are gamma rays. The most customary element is an iron isotope, iron 57 (57Fe). It may generate and absorb radiation of 14.4 keV with a lifetime of 141 ns. Spectroscopy with this type of radiation is called Moessbauer-spectroscopy after the scientist Rudolf Moessbauer, who got the nobel prize for its discovery in 1961. The time scale of 141 ns corresponds roughly to an energy resolution of only 5 nanoelectronvolts. Everything faster than this timescale or everything that transfers an amount of energy larger than this 5 neV changes the spectra. In the early 70th Fritz Parak discovered something that puzzeled theorists over many years: broad lines appeared in Moessbauer absorption spectra of the iron containing protein myoglobin. Although broad lines occur when iron diffuses freely in viscous solution, the broad lines from myoglobin could not be described by this because they appear together with the narrow lines on top. The conclusion was that the iron diffuses in restricted space being linked to segments of the protein molecule. The heme iron in the myoglobin is a probe for protein dynamics and it senses large range motions that occur specificaly in biological macromolecules. It is believed that they are these motions that are responsible for the catalytic and functional activities of the proteins.
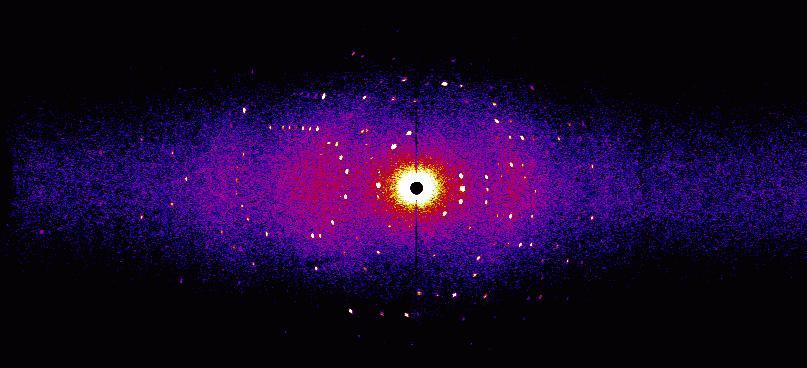
Neutron Crystallography
Hydrogen atoms have a very small scattering cross section for X-rays since they have only one electron and that is mostly on the heavier atom to which the hydrogen is bound. To see the hydrogen, very high resolution X-ray crystallography must be used. However, neutrons are scattered by the nucleus, they are insensitive to electrons and they have a quite large scattering cross section for hydrogen and its heavy sibling deuterium. Using neutron crystallography, the entrire hydrogen network can be observed in a biological macromolecule. In almost all enzymatically catalyzed reactions hydrogen atoms are involved. Neutron crystallography helps to determine the catalytic mechanism of these enzymes. Depicted here is a neutron diffraction pattern of myoglobin (courtesy of A. Ostermann, FRMII TUM).
Time-Resolved Microspectroscopy
Depicted here are spectra from crystals of the protein catalase which were accquired during exposure to X-rays at cryogenic temperatures (100 K). Spectra (a) (d) and (g) are from different catalase species. In (a) the 6th coordination site of the central heme iron is occupied by ammonia, in (d) the central iron is 5-coordinated and in (g) a nitric oxide (NO) is bound to the iron. The NO complex is really remarkable since catalase usually looses NO extremely fast, but here we managed to bind a substantial amount to the crystals using an NO generator (DEANO) in solution. With this we were able to manipulate the crystals at ambient athmosphere without loosing too much NO. The spectral changes during X-ray radiation are clearly visible. (b), (e) and (h) show difference spectra of the corresponding species. In the last row the difference spectra are integrated and plotted as a function of time.