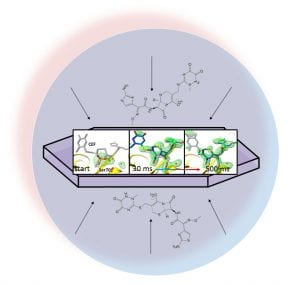
This is the essence of ultrafast structure determination. You see on the left difference electron density near the para-coumaric acid (pCA) chromophore that is bound to the photoactive yellow protein (PYP). This difference electron density has been determined 250 femtoseconds after a reaction has been initiated in PYP crystals by an ultrafast blue laser flash. Red is negative difference electron density, green is positive difference electron density. The electron density of the the chromophore is shifted from red to green as a reaction to the laser flash. However, initially, at 250 fs, the chromophore only buckles and bends back. This is illustrated by the two structures, one in yellow, the other in pink. The yellow structure is the unperturbed dark state structure near the pCA chromophore. The pCA chromophore itsself is flat and lies in one plane. The pink chromophore head (the hexagonal ring) moves a bit downward, and the foot is bent back (feature beta) and lies outside (on the backside) of the plane of the yellow chromophore. However, as one can easily see, the configuration is still trans and similar to that of the yellow chromophore. On the right, you see the structural evolution 3 ps after the laser flash. Again, red is negative and green is positive difference electron density, and the yellow structure is the one of the uperturbed dark state. This time, however, the green structure is different from the pink structure on the left. The chromophore head (the hexagonal ring) is leaning forward, and the foot aligns along one axis. The foot carbonyl (the red oxygen) points outside the plane. This structure is cis. You experience the trans to cis isomerization. There are Nobel prizes for theoretical simulations of these chemically extremely important cis trans isomerizations. We observe it, for the first time ever, directly by shifting electron density clouds, and on the fly!!!, ultrafast, in extreme slow motion.
The ‘mix-and-inject’ principle. Substrate is added to ultra-small enzyme crystals. Diffusion of substrate into the crystals is faster than the turnover time of the enzymes. It is not really clear what is more important, ultrafast structure determination where the reaction is started with an ultrafast laser pulse, or the structural characterization of enzymatically catalyzed reaction on much slower time scales. The former is academically extremely interesting, as it allows to assess new physics and chemistry that noone has seen before. The latter is bio-medically more relevant, since enzymes are potential drug targets, and catalysis is poorly understood. Whatever time scale, the XFEL makes is possible.
August 2024: The Schmidt lab enters the realm of single particle cryo-EM. Driven by limited relaxations in protein crystals and by difficulties to crystallize large macromolecular constructs, we approached the New York Structural Biology Center (NYSBC) for help with single-particle cryo-EM. After we send samples that are typically prepared in collaboration with the Stojkovic lab at Northeastern Illinois University in Chicago, researchers at NYSBC prepare the cryo-EM grids and collect cryo-EM images. At the University of Wisconsin Milwaukee, graduate student Tek Malla established a pipeline for single-particle cryo-EM data processing with cryoSPARC using Prof. Peter Schwander’s compute cluster. More recently, the pipeline is managed by the High Performance Computing Service at UWM and is available to us and all interested users at UWM. Below are cryo-EM densities and structures we obtained for bacteriophytochromes. This established the first ab-initio single-particle cryo-EM structure determination by the Schmidt lab and it is also the first for the entire UWM. The results are published: https://www.science.org/doi/10.1126/sciadv.adq0653.
from 2018 to 2022: We showed with two transforming beamtimes at the European XFEL (EXFEL) how (i) pump-probe type time-resolved crystallographic experiments are feasible and (ii) how mix-and-inject experiments are performed at high repetition rate XFELs. The mix-and-inject experiment was even more transformative than the pump-probe experiment, since it explored the single millisecond time-scale of an enzymatic reaction that was not accessible to these types of experiments previously. We are now able to pursue structure based enzymology at the LCLS and the EXFEL. There is still a lot of scepticism, but there is also overwhelming interest in the function of enzymes and the applicability of this method. We are now ready to push the mix-and-inject method even further with new data collected at the LCLS.
May 31, 2018: BMC-Biology gives birth to our newest baby, mix-and-inject serial crystallography on a beta-lactamase. We characterize the enzyme-substrate complex with the cephalosporin antibiotic Ceftriaxone, as well as the covalently bound acyl adduct where the beta-lactam ring is open, and the Ceftriaxone is chemically altered and covalently bound.
May 6, 2016: Science magazine publishes another paper with UWM postdoc Kanupriya Pande, now Center for Free Electron Lasers (CFEL) in Hamburg, as first author. This is even flashier than the first Science paper, because it goes far beyond proof of principle. We were the very first ever to structurally characterize a trans to cis isomerization, which is a hugely important reaction in Chemistry and Biology. This is possible because proteins provide a soft cushion that allows the reaction to occur without affecting the surrounding of the biological macromolecule. This is then the reason why the crystal lattice stays completely intact, and the reaction can be observed with time-resolved crystallography. However, we had to go to extreme means, and use the fastest camera in the world, the Linac Coherent Light Source, which provides camera frames of 100 femtoseconds. Since the trans to cis isomerization happens at about 550 fs we could make a movie of this transition in ultra slow motion. As quantum molecular mechanics simulations suggest, the trans to cis isomerization proceeds through a socalled ‘conical intersection’, which is the seam where the electronic ground state potential energy surface meets the electronic excited state potential energy surface. Our experiments show for the first time, directly and on-the-fly, the structural transition through such a conical intersection, which has been sought after for a long time.
Jan, 2016: Schmidt lab researchers are co-authors on a Nature paper that has the potential to push crystallography to a new frontier by going beyond Bragg scattering. In this paper, diffuse scattering (‘diffusion diffuse’ in French thanks to one of my colleagues here), is used to extend resolution far beyond the highest resolution Bragg reflections. This has been unheard of. The publication is led by the Chapman group at CFEL (Hamburg), and its first author is K. Ayyer from CFEL. Now, there exists an opportunity to use even relatively bad crystals, and collect high resolution data from them.
Dec 5, 2014: Science magazine publishes a paper with Jason Tenboer, Schmidt lab graduate student, as first author. It seems as if this is the first Science paper with a UWM first author since 1988. It also seems that it is the first Science paper with a UWM corresponding author since 1988. There are four additional UWM affiliates on this paper: Dr. Kanupria Pande, Prof. Abbas Ourmazd, Jennifer Scales, Prof. Peter Schwander. This paper reports that time-resolved serial femtosecond investigations at near atomic resolution are possible. The quality of the difference electron density maps obtained equals and even exceeds that obtained at synchrotron light sources. The Schmidt lab celebrates.
Aug 18th, 2014: Schmidt starts the semester as Full Professor.
May 18th, 2014: The Schmidt lab dislocated to a remote place in Menlo Park, Ca to perform an experiment at the world’s brightest light source for hard X-rays, the Linac Coherent Light Source (LCLS).
November 6th, 2013
The news is out: We are members of a National Science Foundation Science and Technology Center called Biology with X-ray Lasers, BioXFEL
The center consits of 8 universities with about 20 groups that are aiming at the determination of structures of biomolecules from tiny crystals down to the single molecule level. The STC is the most prestigious award of the NSF to a group of investigators. We all are proud to be part of it!!!
From the NSF web-page:
“The Science and Technology Centers (STCs): Integrative Partnerships program supports innovative, potentially transformative, complex research and education projects that require large-scale, long-term awards. STCs conduct world-class research through partnerships among academic institutions, national laboratories, industrial organizations, and/or other public/private entities, and via international collaborations, as appropriate. They provide a means to undertake significant investigations at the interfaces of disciplines and/or fresh approaches within disciplines. STCs may involve any areas of science and engineering that NSF supports. STCs investments support the NSF vision of advancing discovery, innovation and education beyond the frontiers of current knowledge, and empowering future generations in science and engineering.”
July 2013, we (K. Pande, D. Saldin) show with theoretical calculations and computer simulations that time-resolved structures of reaction intermediates can be retrieved at near atomic resolution (2 -3 A) solely from solution scattering. The Schmidt lab will run experiments.
July 2013, Schmidt and Pacheco labs joint NSF fund is extended by another three year. Fantastic!
July 2012, M. Schmidt hosts the session “Emerging X-ray sources, theory and practice I” at the ACA meeting in Boston. Invited speakers include F. Schotte, J. Tainer, P. Schwander, D. Saldin, P. Musumeci, H. Lemke.
May 2012, D. Saldin and M. Schmidt receive NSF funding to explore theory and practice of “time-resolved atomic structure determination from solution”.
October 2011, PI Schmidt receives UWM graduate school research award.
July 2011, Schmidt and Pacheco labs receive word from the National Science Foundation, that they will receive funds for another 2 years for their project “Structure-function relationships in metalloenzymes with multiple redox centers”.
July 2011, PI Schmidt participates in the UW-Milwaukee “Research Experience for Teachers” (RET) program funded by the NSF.
July 2011, the Schmidt lab hosts an American Chemical Society (ACS) SEED student for the second time.
February 2011, PI Schmidt is recommended for promotion to Associate Professor with Tenure by the UW-Milwaukee Division of Natural Sciencies Executive Committee.
July 2010, The Schmidt lab participates in the American Chemical Society (ACS) SEED Program.
The ACS approves the inclusion of the Schmidt lab in its SEED program. The SEED program supports K12 high school student from a minority background for a summer internship in a research lab. From the ACS web page: “The ACS Project SEED summer research program opens new doors for economically disadvantaged students to experience what it’s like to be a chemist. Students entering their junior or senior year in high school are given a rare chance to work alongside scientist-mentors on research projects in industrial, academic, and federal laboratories, discovering new career paths as they approach critical turning points in their lives”.
February 2010, Marius Schmidt receives prestigious NSF Career award.
5-Dimensional crystallography is supported by NSF’s prestigious young investigator award starting in March 2010. We will determine comprehensive kinetic mechanisms including entropy, enthalpy and prefactors of all rate coefficients that determine the mechanism by varying the temperature.
July 2009, A comprehensive time-series of time-resolved X-ray data from only one crystal.
In July 2009 we collected at BioCARS 14-IDB, Advanced Photon Source, Argonne National Laboratory, U.S.A., 28 time-resolved Laue data sets from only ONE crystal of photoactive yellow protein of diameter 80 um and length ~1mm within only 6.5 hours of synchrotron beam time. Structure factor amplitudes were determined from the raw diffraction pattern in only 2 days using Precognition/Epinorm (RenzResearch). The comprehensive time-series spans a time interval from 4 ns to 256 ms with time-points equidistantly arranged in logarithmic time. The difference electron density was analyzed globally by the singular value decomposition to extract the kinetics.