A controversial application of proton therapy is prostate cancer treatment. To spare radiosensitive rectal tissue the standard prostate cancer protocol treats only with horizontal beams, which travel through thick bony structures before reaching the prostate. Bone degrades the proton beam’s energy spectrum, minimizing the advantage of proton therapy over intensity modulated x-ray radiation therapy. Horizontal-only delivery maximizes proton path length through hip and thighbones. Accurate range verification could enable delivery using oblique beams that avoid large bones and maintain a sharp Bragg peak, taking full advantage of the potential of particle therapy. Therefore, we performed a Monte Carlo simulation study and developed a two-step method for accurately estimating the Bragg peak location from thermoacoustic emissions measured by receivers that could be embedded onto the form factor of a transrectal ultrasound imaging array (Fig. 1, Med Phys 45(2):783-793).
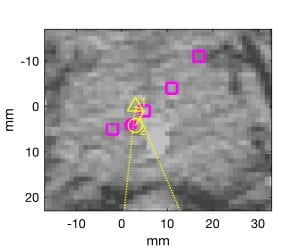
Fig. 1. Monte Carlo simulations of oblique beam and range estimates from SNR=0 dB signals overlaid on planning CT. “+” indicate locations of low frequency receive elements projected orthogonally onto each CT plane. Axial, sagittal, and coronal planes through true Bragg peak location in (a) through (c), respectively. White line in (a) and (d) indicates beamline. Magenta dot is true Bragg peak location. Yellow triangle and circle indicate orthogonal projections of beamformed and final estimates. (d) Zoomed image of axial plane near Bragg peak. CT display window is [-200, +200] HU.
RIGHT: Bragg peak locations for “control” beams and “measured” beam are plotted with magenta squares and dot, respectively. Dotted yellow lines connect the transducer locations to the beamformed estimate (yellow triangle). A dashed line connects the beamformed estimate to the nearest control point, and a solid yellow line connects the control point to the final estimate (yellow circle).
Experimentally, we collected preliminary data by propagating 50 MeV protons into a waterbath and gelatin phantom designed to test sensitivity to anatomic change. Monte Carlo simulations predicted 21.1 mm range in water; thermoacoustic range estimates in Table 1 were accurate to within 1 mm. (Phys Med Bio 61, pp. 5621-5638).
Table 1. Water Range Estimates
| 1 cm left | centered | 1 cm right |
| 21.7±0.2 | 21.9±0.0 | 22.0±0.02 |
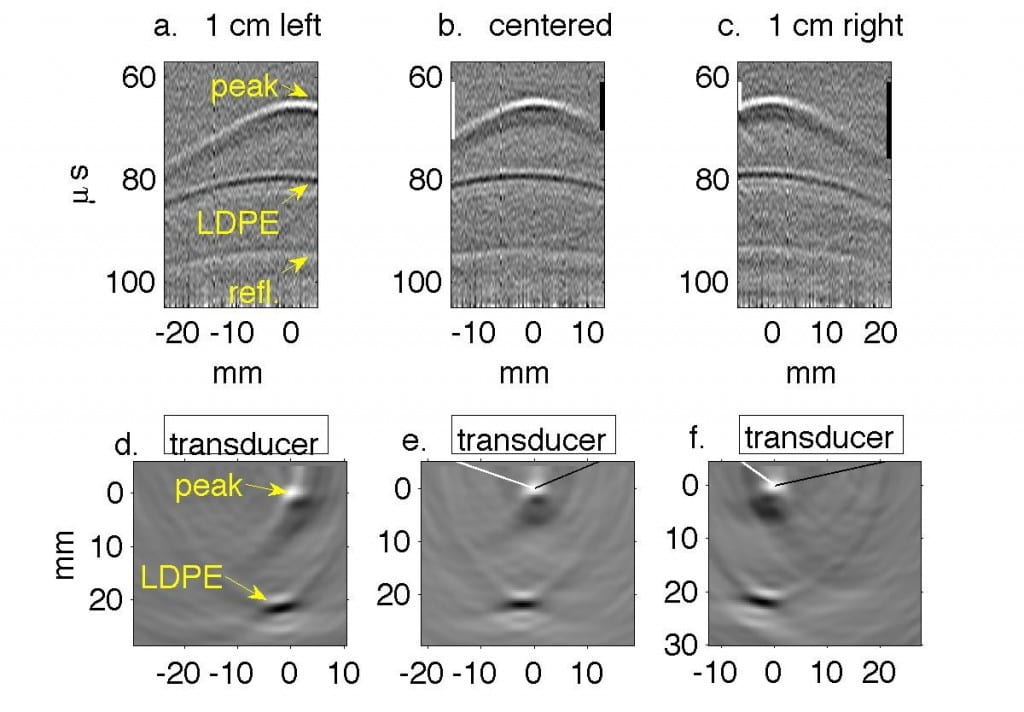
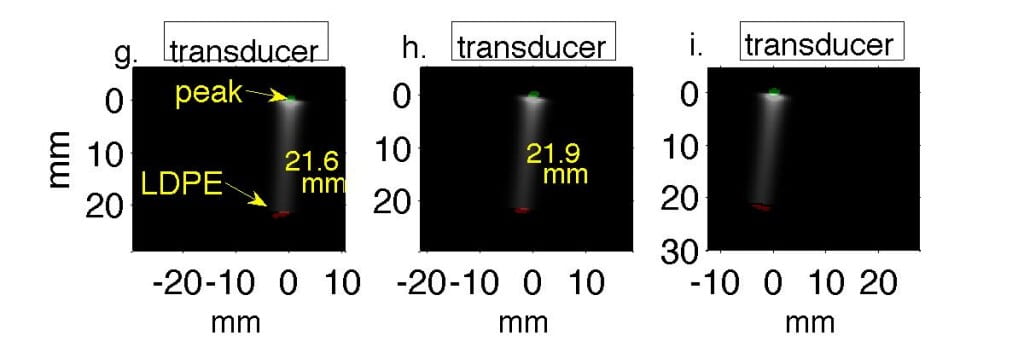
Fig. 2. Water tank results in which transducer is translated in 1 cm increments perpendicular to the beamline. In Figs. a, d, g the transducer axis is 1 cm left of the beamline. In Figs. b, e, h the transducer is centered on the beamline, and in Figs. c, f, i it is 1 cm right of the beamline. (a-c) Filtered P4-1 data, with timing measured from chopper plate discharge, not entry of beam into target. Horizontal axes represent channel number. (d-f) Reconstructions of the data reveal the Bragg peak as a monopole 6.5 mm from the transducer. The proximal end of the beam as it enters the LDPE appears as a bipolar mark. (g-i) Regions of strongest and weakest pressures from (d-f) overlaid onto Monte Carlo simulations in green and red, representing Bragg peak and LDPE, respectively.

Fig. 3. Grayscale ultrasound image with Bragg peak overlaid in yellow; beam entry point into high stopping power target in red. First realizations. (a) sagittal, cavity filled with olive oil. (b) sagittal, cavity empty. (c) coronal, cavity filled with olive oil, but thermoacoustic images from both empty and oil-filled experiments are overlaid. (d) x-ray CT of phantom. coronal slice in which beam traveled upwards through styrofoam cone and cavity.
During phantom testing, the beam either stopped in an oil-filled cavity or flew unimpeded through an empty cavity into gelatin. Bragg peaks were detected at depths from the transducer of nearly 3 cm and more than 6 cm when the cavity was empty and filled with olive oil, respectively. Repeatability was excellent when filled with olive oil, which generates stronger thermoacoustic emissions than gelatin.