see recent results on thermoacoustic range verification https://www.linkedin.com/company/acoustic-range-estimates/?viewAsMember=true
Algorithm development for direct image reconstruction is my area of training, although I now collect experimental data. My current research is on applications of thermoacoustics, a hybrid technique in which rapid heating induces outgoing pressure pulses that are detected noninvasively by transducers outside the field of view.
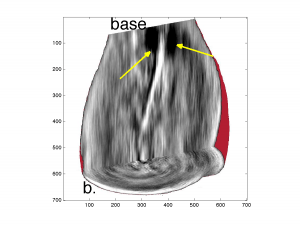
Thermoacoustic imaging of electrical conductivity in human prostates (Fig. 1) was performed in my lab here at UWM; thermoacoustic range estimates have been computed from both simulated and measured data.
My early mathematical research in diffuse tomography was motivated by optical/NIR imaging, followed by cone beam reconstruction of xray CT data and motion correction for Propeller MRI during my eight years with General Electric (GE). Although my degrees are in applied mathematics, at GE I obtained a basic understanding of the physics—and painstaking engineering—required to develop clinical systems.
Fig. 1. Thermoacoustic images of a human prostate. Visualization of data collected by a cardiac ultrasound array where the compressed urethra indicated by yellow arrows descends towards the apex (a) and seminal vesicles are indicated by yellow arrows (b). Quantitative reconstruction of induced pressure and electrical conductivity (c). (IEEE TUFFC, 63(2), pp. 245-55).
More recently, we demonstrated experimentally that when thermoacoustic emissions are detected by receivers collocated with an ultrasound imaging array range estimates are robust to acoustic heterogeneity relative to the ultrasound image of underlying morphology.
Thermoacoustics could provide online range verification during particle therapy with direct correlation to underlying morphology as depicted in ultrasound images (Fig. 2), and without exposuring organs at risk to ionizing radiation. Incorrect soundspeed settings and acoustic heterogeneities dilate and deform ultrasound images respectively. Recently, we demonstrated that thermoacoustic range estimates are subject to the same transformations as ultrasound images when thermoacoustic receivers are co-located with ultrasound imaging arrays. Thermoacoustic emissions traveled more than 10 cm through oil in which a strong reflector (5 mm bone-mimicking sample) and diffuse reflector (tissue mimicking gelatin) were placed to obstruct transmission. Nevertheless, thermoacoustic emissions were detected by the ultrasound transducer array, and used to recover not only the location of the beam’s Bragg peak, but also the beam range into the target and location of the entry wall into the target. The estimated entry window location agrees with the ultrasound image, and the range of the beam agreed with Monte Carlo simulations to within 300 microns. 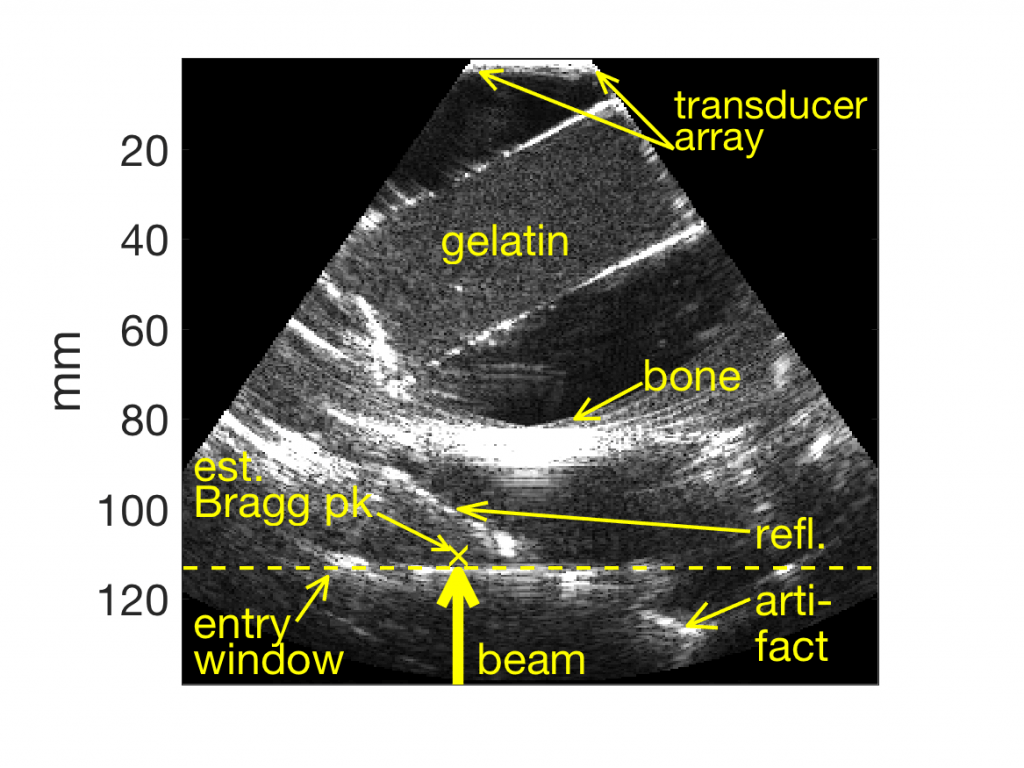 Fig. 2. Ultrasound image of acoustic scatterers in an oil bath. A beam of 16 MeV helium ions traveled upwards through an acrylic entry window and into the oil. Thermoacoustic range estimates of Bragg peak location and ion entry window estimated from a single 4He pulse are overlaid as dashed line and yellow ‘x’.
Fig. 2. Ultrasound image of acoustic scatterers in an oil bath. A beam of 16 MeV helium ions traveled upwards through an acrylic entry window and into the oil. Thermoacoustic range estimates of Bragg peak location and ion entry window estimated from a single 4He pulse are overlaid as dashed line and yellow ‘x’.