
Revised conceptual model in cross-section of three zones containing elevated concentrations of solid-phase uranium and the associations of uranium with respect to the median water table level and geochemical conditions, e.g., gypsum-rich, vadose-rich, and organic-rich zones and the likely mobilization mechanisms, e.g., dissolution, desorption, and flushing, during recharge of river water (Paradis et al., 2020).
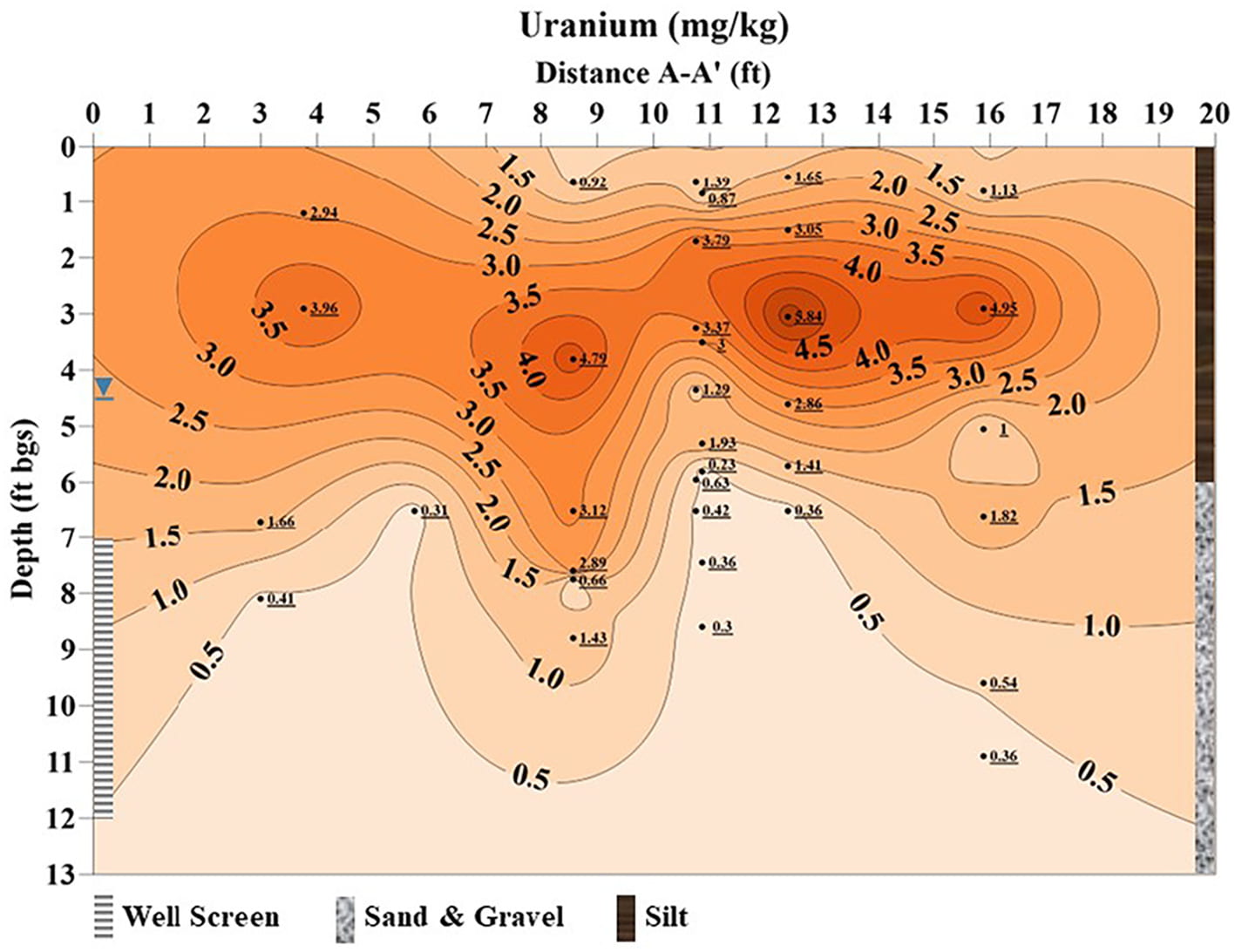
Pre-test isoconcentration profile map of uranium (mg/kg) on aquifer sediments (5% nitric acid leached) above and below baseflow water table (≈ 4.5 ft bgs, ≈ 1.4 m bgs) along A to A’ transect (Fig. 4), bgs = below ground surface, distance from A to A’ is 20 ft (6.1 m), depth is 13 ft (4.0 m) (Paradis et al., 2022).
Uranium (U) is a redox-sensitive element whose mobility in its oxidized state (U(VI)) is typically greater than in its reduced state (U(IV)). The mobility of uranium can also be affected by pH, alkalinity, presence/absence of clays and/or organic matter, and the physical nature of porous media, e.g., single-porosity granular versus dual-porosity fractured porous media. The objective of the uranium mobility research is to characterize the mechanisms, e.g., advection, molecular diffusion, mechanical dispersion, sorption/desorption, precipitation/dissolution, and matrix diffusion that control its mass transport to better inform predictive numerical models at select sites associated with the United States Department of Energy Office of Legacy Management.
The Paradis lab recently published its first peer-reviewed scientific article from research funded by Legacy Management at the Grand Junction site in Colorado, entitled, “Field experiments of surface water to groundwater recharge to characterize the mobility of uranium and vanadium at a former mill tailing site“. The Paradis lab plans to conduct similar research at the Riverton site in Wyoming in the summer of 2020.
Graduate students Cullen Meurer and Jiyan Hatami presented their uranium mobility research at GSA’s North-Central Section 54th Annual Meeting in 2020. Cullen’s presentation was entitled, “Enhanced mass reduction of uranium via alkalinity-driven desorption and groundwater flushing” and Jiyan’s presentations was entitled, “Sorption of 2-naphthalene sulfonte to organic-rich sediments in batch and column experiments“.