Nanoscale science, engineering, nanotechnology and its applications in energy storage, water-energy nexus, catalysis, bioengineering, molecular detection and electronic devices. In particular, lithium-metal battery, lithium-silicon battery, advanced lead-acid battery, and the electro-chemo-mechanical diagnosis using in-situ technique. The areas include:
- Anode and cathode electrode materials in lithium-metal/silicon batteries for electric vehicles (EVs)
- Solid electrolyte in all solid-state batteries
- Water treatment technology in removing PFAS/bacteria/metal ions contaminations from groundwater, drinking water and wastewater.
- Superhydrophobic/hydrophilic materials as self-cleaning coating
Research Summary
Thermal Heated S/C Composite as Cathode
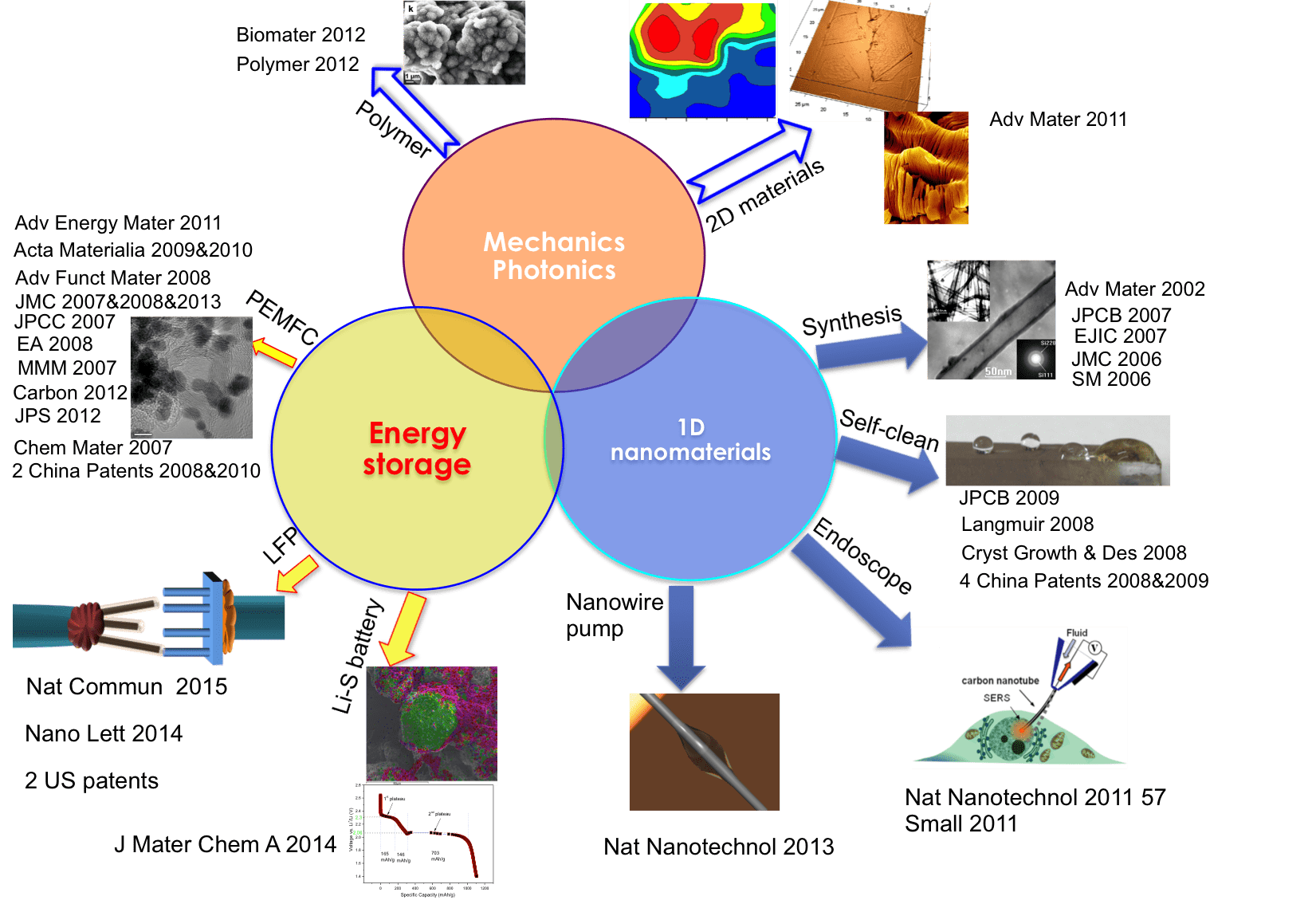
For the cathode electrode materials, we have developed a lithium-sulfur double-nets composite (SULFUN) cathode with improved cyclability using a low-cost and reliable synthesis method (Figure 3) (J Mater Chem A, 2 19788, 2014). The industrially available conductive carbon black was used as the carbon matrix (Cnet) and was infused with sulfur (Snet) to form core-shell-like networks (Cnet + Snet). Without the network protection, the big commercial sulfur particles will lose capacity quickly. As a result, the 520 mAh/g and 290 mAh/g discharge capacities were attained after 300 and 500 cycles, respectively (Figure 4). These data indicate that the porous matrix can prevent the dissolution of intermediate products from S/C nanocomposite electrode.
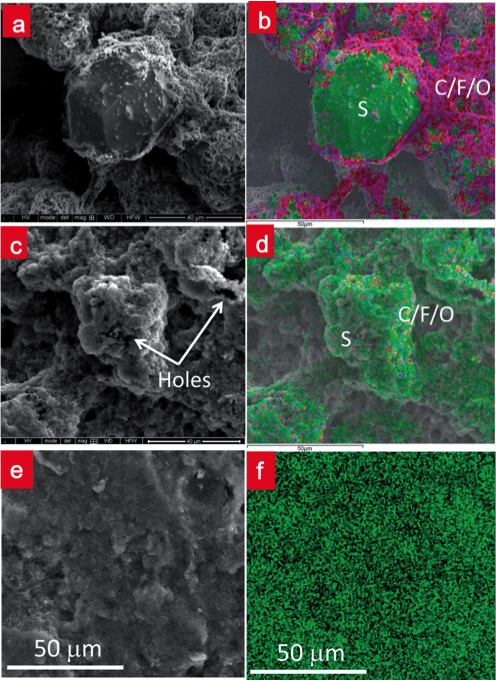
Figure 3. Morphology evolution of commercial sulfur particle and SULFUN during the charge–discharge. SEM morphologies of the commercial sulfur mixture with Super-P® and binder before (a) and after (c) two rounds of cycling. (b) and (d) EDS element mappings of the area shown in (a) and (c), respectively. (e) SEM morphology of the electrode made of the SULFUN annealed at 200 °C for 2.5 hours after two cycles. (f) The corresponding sulfur element mapping from (e).
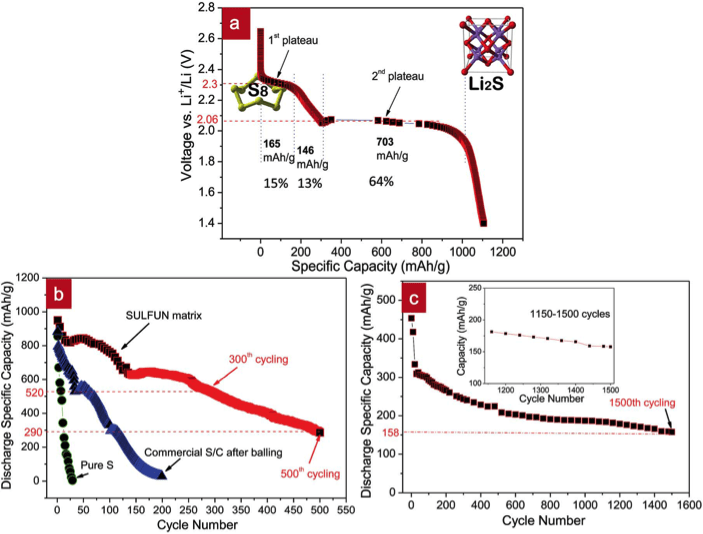
Figure 4. Discharge voltage profiles of SULFUN matrix. Discharge voltage profile of the matrix treated at 200 °C with 2.5 hours at a rate of 0.2 C (a). Cycling performance of commercial pure sulfur (green), mortar milled S–C mixture (blue), and SULFUN matrix (red) treated under 200 °C for 2.5 hours (b) and 150 °C for 50 hours (c) at a rate of 0.2 C.
In-situ TEM of LiFePO4 Nano-Battery
We built a nano-battery (Figure 5) and observed the real electrochemical energy conversion taking place in situ inside a TEM, using a single LFP nanowire (Nano Letters, 14 4005, 2014). Figure 5a shows the schematic of such a nano-battery, consisting of a single LFP nanowire as the working electrode, electrolyte, and Si nanowire as the cathode. The whole nanobattery can be placed into TEM, enabling real-time imaging of the Li charging and discharging processes. The nano-battery also allows direct investigation of the intrinsic behavior of the nanoelectrodes without interference from the liquid.
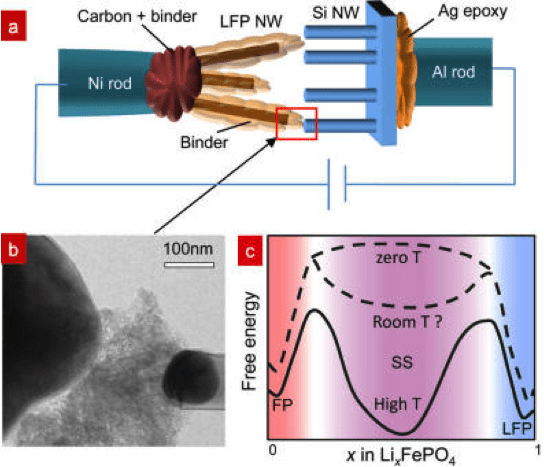
Figure 5. In-situ TEM observation of LFP nanobattery and the free energy graph. (a) Experimental setup inside TEM. (b) TEM image of the contact between LFP NW and SiNW. (c) Schematic of free energy versus composition g(c) for FP, SSZ, and LFP.
LiFePO4 as an electrode material has attracted great interest in the Li-ion battery community. Debates on the presence and roles of a solid solution zone (SSZ) in lithiation/delithiation originated from the apparent high rate capability of LFP batteries despite the poor electronic/ionic conductivities of bulk LiFePO4 and FePO4. A potentiostatic in-situ TEM technique was used to study the LiFePO4electrode kinetics during delithiation. Based on real-time, high-resolution TEM analysis, SSZ forms quickly and reaches 10-25 nm × 20-40 nm on ac face, which directly contacts the electrolyte, in lieu of sharp LiFePO4→FePO4 interfaces observed in some other situations (Figure 6). This 20nm scale SSZ was apparently quite stable and persisted for hundreds of seconds during the experiment. Compared with the atomically sharp LiFePO4→FePO4 interface, the wide SSZ seen here contains no dislocations, so reduced fatigue, enhanced cycle life, and enhanced rate capability can be expected. These findings suggest that SSZ can dominate electrode transformation when the particle size reaches sub-100nm; for larger particles, SSZ could still be important during fast charging, as it provides out-of-equilibrium but atomically wide avenues for Li+/e- transport (Figure 7).
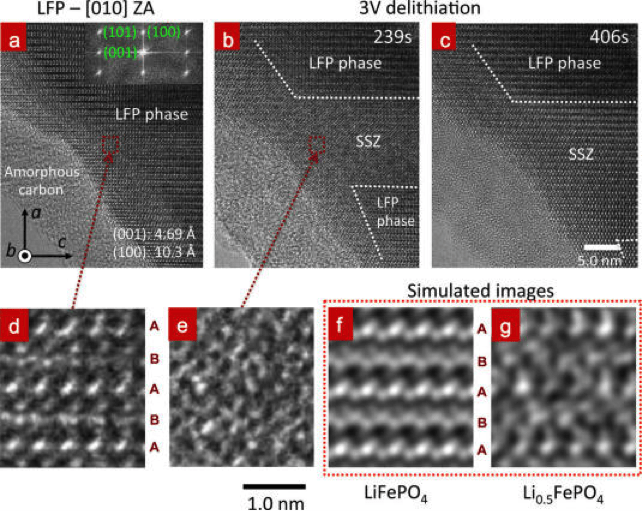
Figure 6. Delithiation process of a LFP crystal viewed from [010] zone axis using SiNW as anode electrode under +3 V (a−c). (a) The LFP has a crystal structure before applying the voltage. The FFT (inset) indicates lattice spacings of (100) and (001), which are 10.3 and 4.69 Å. (b) A SSZ of 11~22 nm2 and a boundary propagating along a direction were developed at 239s. The boundary of SSZ/LFP is marked by the dashed lines. (c) The SSZ and boundary evolution at 406s. (d,e) The magnified images of the dotted red rectangle in (a,b). The simulated TEM images of (f) LiFePO4and (g) Li0.5FePO4 are shown for comparison.
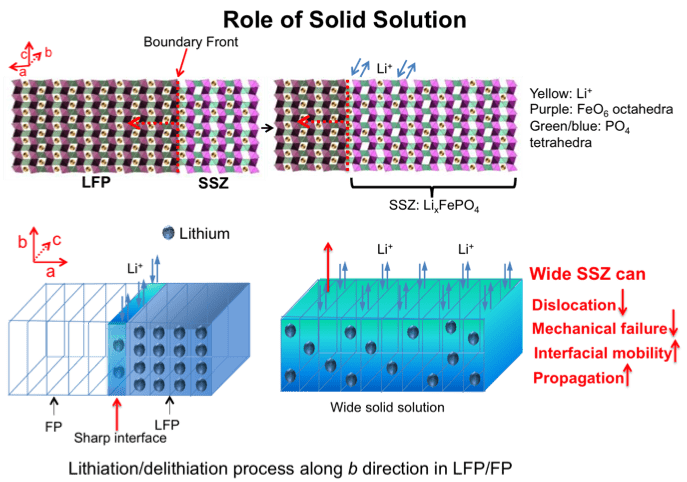
Figure 7. Role of solid solution in LFP.
Intracellular Detection of Molecules
We aim to develop a functionalized NW/NT endoscope that has the ability to provide fingerprint data of electrochemistry and spectra upon intracellular probing of a single cell. With high sensitivity and high selectivity, the 1D endoscope can focus on a small intracellular domain in a minimally intrusive way to record the redox reaction dynamically via electrochemical testing in a living cell real time. The endoscope can also identify the component change and oxygen consumption even with trace amounts inside via the enhanced spectra and electrochemical signaling, to further define the variation, damage and metabolism functions of the cell. The multi-functional endoscope can also be used for intracellular nano-cargo delivery. The manipulation and diagnostics of an individual cell can help us develop deep understanding of the fundamental function and mechanism inside the cell. The technique shows great potential for early cancer detection and post-cancer treatments.
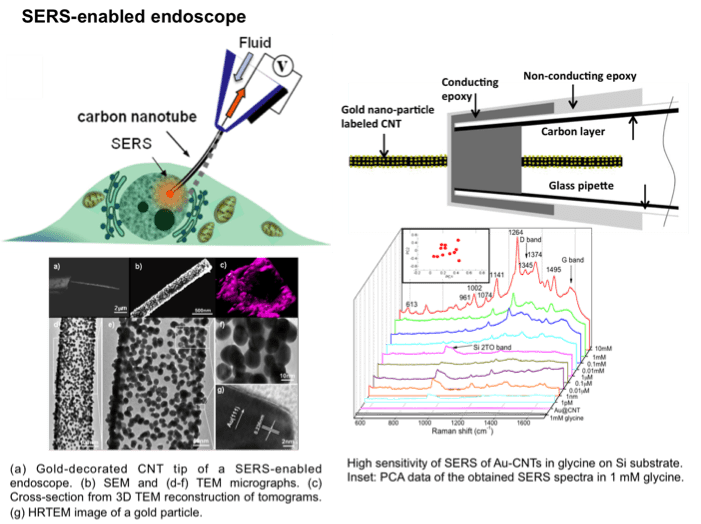
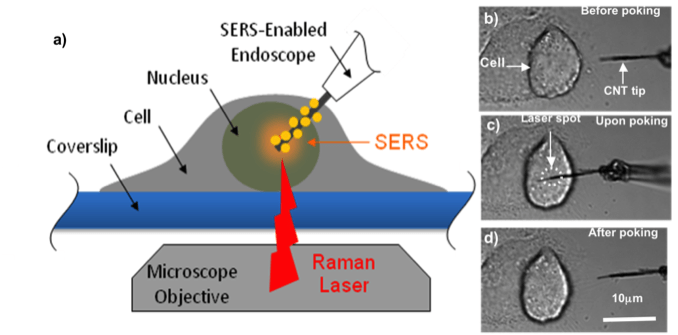
(Niu et al. Small, 7 540, 2011; Nature Nanotechnology, 6 57, 2011)