Research Statement
Neuroscience historically stems from human curiosity to unravel the deepest mysteries of himself to address questions such as the meaning of life, intelligence, creativity, or even consciousness. Over centuries, the scientists who devoted their lives to understanding of the brain and the mind came from a variety of different disciplines, from biology and psychology to mathematics, physics, and engineering. Nonetheless, the scientific approach to understanding the structure, function, biochemistry, and physiology of the nervous system is a relatively new endeavor. Brain is an assembly of billions of coupled nerve cells where each cell functions as a nonlinear complex processing element. Subpopulations of neurons build up self- organizing networks or topographic computational maps that develop over time and adapt for certain parallel data processing. To unravel mysteries of signal processing in the brain, we should build a profound image of the structure and functionality of such networks. This knowledge leads to: 1. Promoting our understanding of nervous system, brain functionalities, and discovery of new treatments for psychiatric disorders, 2. Engineering systems that compensate or bypass injured/dysfunctional nervous circuits for neuroprosthetics and brain- machine interface (BMI) applications, 3. Complementing the architecture of conventional computers by adding the superiority of performing higher-level operations such as perception, cognition, and intelligent acts. Understanding the brain requires advanced technologies for imaging and modulation/recording of neural activity. Currently, we have focused on development of new paradigms to control and simultaneously image neural activity in large-scaled networks of the brain using the tools of photonics, electronics, and molecular genetics. BIST Lab is the second home for a group of young investigators with diverse educational backgrounds that work together as a team to develop new instrumentation and methodologies for the study of nervous system and the brain. We use enabling technologies such as electronics, optics, electromagnetics, photonics, plus signal processing and software engineering to implement our new ideas for brain functional imaging or modulation of neural activity to study the dynamics of the brain microcircuits. Activities at BIST Lab covers cross disciplinary fields including engineering, mathematics and physics, neuroscience, molecular genetics, and computational neuroscience.
Brain Research
Our research agenda is all about using our engineering skills to develop new instrumentation for the study of the brain. We try to understand how the brain processes information and how we can control, manipulate, and monitor neural, astrocyte, and hemodynamic activities in the brain.
Optogenetic Brain Interfaces

 Optogenetics is a powerful tool in the study of the brain. In this techniques, neurons in the region of interest within the brain tissue are genetically modified to become sensitive to light. Once the tissue is light sensitive, we control and manipulate neural activity by exposing the area to light of appropriate wavelength(s). Part of our research is concentrated on development of new methodologies to control the distribution of light inside the 3D structure of the brain and also simultaneously image induced activities in the brain.
Optogenetics is a powerful tool in the study of the brain. In this techniques, neurons in the region of interest within the brain tissue are genetically modified to become sensitive to light. Once the tissue is light sensitive, we control and manipulate neural activity by exposing the area to light of appropriate wavelength(s). Part of our research is concentrated on development of new methodologies to control the distribution of light inside the 3D structure of the brain and also simultaneously image induced activities in the brain.
Optogenetic Brain Interfaces
 The brain is a large network of interconnected neurons where each cell functions as a nonlinear processing element. Unraveling the mysteries of information processing in the complex networks of the brain requires versatile neurostimulation and imaging techniques. Optogenetics is a new stimulation method which allows the activity of neurons to be modulated by light. For this purpose, the cell-types of interest are genetically targeted to produce light-sensitive proteins. Once these proteins are expressed, neural activity can be controlled by exposing the cells to light of appropriate wavelengths. Optogenetics provides a unique combination of features, including multimodal control over neural function and genetic targeting of specific cell-types. Together, these versatile features combine to a powerful experimental approach, suitable for the study of the circuitry of psychiatric and neurological disorders. The advent of optogenetics was followed by extensive research aimed to produce new lines of light-sensitive proteins and to develop new technologies: for example, to control the distribution of light inside the brain tissue or to combine optogenetics with othermodalities including electrophysiology, electrocorticography, nonlinearmicroscopy, and functional magnetic resonance imaging. (Read more in our IEEE RBME paper)
The brain is a large network of interconnected neurons where each cell functions as a nonlinear processing element. Unraveling the mysteries of information processing in the complex networks of the brain requires versatile neurostimulation and imaging techniques. Optogenetics is a new stimulation method which allows the activity of neurons to be modulated by light. For this purpose, the cell-types of interest are genetically targeted to produce light-sensitive proteins. Once these proteins are expressed, neural activity can be controlled by exposing the cells to light of appropriate wavelengths. Optogenetics provides a unique combination of features, including multimodal control over neural function and genetic targeting of specific cell-types. Together, these versatile features combine to a powerful experimental approach, suitable for the study of the circuitry of psychiatric and neurological disorders. The advent of optogenetics was followed by extensive research aimed to produce new lines of light-sensitive proteins and to develop new technologies: for example, to control the distribution of light inside the brain tissue or to combine optogenetics with othermodalities including electrophysiology, electrocorticography, nonlinearmicroscopy, and functional magnetic resonance imaging. (Read more in our IEEE RBME paper)
Closed-Loop Optogenetic Brain Interface
 This work reports a new approach for implementation of closed-loop brain-machine interface algorithms by combining optogenetic neural stimulation with electrocorticography and fluorescence microscopy. We used a new generation of microfabricated electrocorticography (micro-ECoG) devices in which electrode arrays are embedded within an optically transparent biocompatible substrate that provides optical access to the brain tissue during electrophysiology recording. An optical setup was designed capable of projecting arbitrary patterns of light for optogenetic stimulation and performing fluorescence microscopy through the implant. For realization of a closedloop system using this platform, the feedback can be taken from electrophysiology data or fluorescence imaging. In the closed-loop systems discussed in out recent paper, the feedback signal was taken from the micro-ECoG. In these algorithms, the electrophysiology data is continuously transferred to a computer and compared with some predefined spatial-temporal patterns of neural activity. The computer which processes the data also readjusts the duration and distribution of optogenetic stimulating pulses to minimize the difference between the recorded activity and the predefined setpoints so that after a limited period of transient response the recorded activity follows the setpoints.(Read more in our IEEE TBME paper, TBME Features Story).
This work reports a new approach for implementation of closed-loop brain-machine interface algorithms by combining optogenetic neural stimulation with electrocorticography and fluorescence microscopy. We used a new generation of microfabricated electrocorticography (micro-ECoG) devices in which electrode arrays are embedded within an optically transparent biocompatible substrate that provides optical access to the brain tissue during electrophysiology recording. An optical setup was designed capable of projecting arbitrary patterns of light for optogenetic stimulation and performing fluorescence microscopy through the implant. For realization of a closedloop system using this platform, the feedback can be taken from electrophysiology data or fluorescence imaging. In the closed-loop systems discussed in out recent paper, the feedback signal was taken from the micro-ECoG. In these algorithms, the electrophysiology data is continuously transferred to a computer and compared with some predefined spatial-temporal patterns of neural activity. The computer which processes the data also readjusts the duration and distribution of optogenetic stimulating pulses to minimize the difference between the recorded activity and the predefined setpoints so that after a limited period of transient response the recorded activity follows the setpoints.(Read more in our IEEE TBME paper, TBME Features Story).


Monitoring Cerebral Hemodynamics Following Optogenetic Stimulation via Optical Coherence Tomography
 In this research, spectral domain optical coherence tomography is used to measure the hemodynamic response induced by optogenetic stimulation in the somatosensory cortex of transgenic mice. By analyzing the 3-D angiograms and Doppler measurementsproduced by coherence tomography,we observed significant increase in blood flow as a result of increased vessel diameter and blood velocity following optical stimulation of cortical neurons. Such distinct responses were not observed in control experiments where the brain of wild-type mice were exposed to the same light pulses. (Read more in our IEEE TBME paper).
In this research, spectral domain optical coherence tomography is used to measure the hemodynamic response induced by optogenetic stimulation in the somatosensory cortex of transgenic mice. By analyzing the 3-D angiograms and Doppler measurementsproduced by coherence tomography,we observed significant increase in blood flow as a result of increased vessel diameter and blood velocity following optical stimulation of cortical neurons. Such distinct responses were not observed in control experiments where the brain of wild-type mice were exposed to the same light pulses. (Read more in our IEEE TBME paper).
Spectral-Domain Optical Coherence Tomography
Graphene-based carbon-layered electrode array technology for neural imaging and optogenetic applications
 Neural micro-electrode arrays that are transparent over a broad wavelength spectrum from ultraviolet to infrared could allow for simultaneous electrophysiology and optical imaging, as well as optogenetic modulation of the underlying brain tissue. The long-term biocompatibility and reliability of neural micro-electrodes also require their mechanical flexibility and compliance with soft tissues. Here we present a graphene-based, carbon-layered electrode array (CLEAR) device, which can be implanted on the brain surface in rodents for high-resolution neurophysiological recording. We characterize optical transparency of the device at 490% transmission over the ultraviolet to infrared spectrum and demonstrate its utility through optical interface experiments that use this broad spectrum transparency. These include optogenetic activation of focal cortical areas directly beneath electrodes, in vivo imaging of the cortical vasculature via fluorescence microscopy and 3D optical coherence tomography. This study demonstrates an array of interfacing abilities of the CLEAR device and its utility for neural applications. (Read more in our Nature Communications paper).
Neural micro-electrode arrays that are transparent over a broad wavelength spectrum from ultraviolet to infrared could allow for simultaneous electrophysiology and optical imaging, as well as optogenetic modulation of the underlying brain tissue. The long-term biocompatibility and reliability of neural micro-electrodes also require their mechanical flexibility and compliance with soft tissues. Here we present a graphene-based, carbon-layered electrode array (CLEAR) device, which can be implanted on the brain surface in rodents for high-resolution neurophysiological recording. We characterize optical transparency of the device at 490% transmission over the ultraviolet to infrared spectrum and demonstrate its utility through optical interface experiments that use this broad spectrum transparency. These include optogenetic activation of focal cortical areas directly beneath electrodes, in vivo imaging of the cortical vasculature via fluorescence microscopy and 3D optical coherence tomography. This study demonstrates an array of interfacing abilities of the CLEAR device and its utility for neural applications. (Read more in our Nature Communications paper).
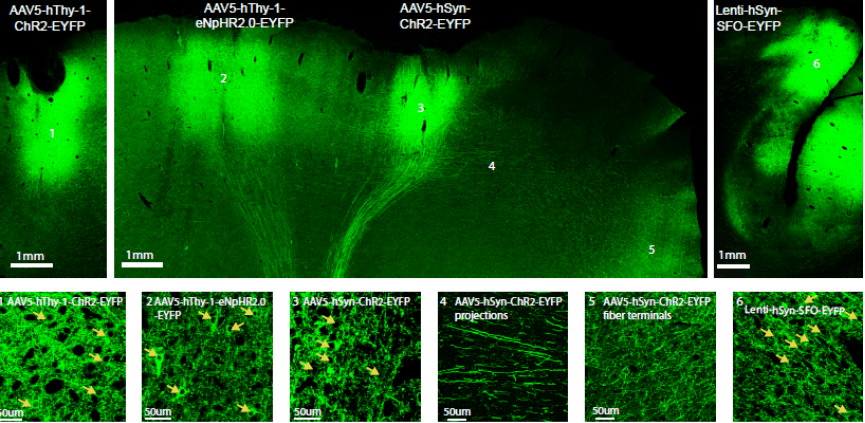
Optogenetic Tools for non-human Primates
Optogenetics is a technique for controlling subpopulations of neurons with light in the intact brain, which complements classical electrophysiology and may enhance both basic systems physiology research as well as inform the mechanisms and treatment of brain injury and disease. Before launching large-scale primate studies, the method needs to be further characterized and adapted for use in the primate brain. We report here an optogenetic toolbox designed for primates, including two optogenetic viral vector systems based on lentiviruses and adeno-associated viruses (AAV), combined with the human promoters hSyn and hThy-1 in rhesus macaques. We also assess the use of three different mammalian codon-optimized opsins in the primate brain: channelrhodopsin-2 (ChR2), enhanced Natronomonas pharaonis halorhodopsin eNpHR2.0, and a step-function opsin (SFO), which we characterize electrophysiologically, histologically, and behaviorally. We show that expression of these opsins is strong under control of the human promoters hSyn and hThy-1, and that low light levels are sufficient to activate or deactivate those opsins as measured on a single cell and local field potential level. We also introduce a new device for measuring in vivo fluorescence over time, allowing the assessment of construct expression levels in the intact brain. Together, we present a set of optogenetic tools designed for optogenetic experiments in the non-human primate brain. (Read more in our Nature Neuroscience paper).
Extraction of Optical Properties and Prediction of Light Distribution in Rat Brain Tissue
Predicting the distribution of light inside any turbid media, such as biological tissue, requires detailed information about the optical properties of the medium, including the absorption and scattering coefficients and the anisotropy factor. Particularly, in biophotonic applications where photons directly interact with the tissue, this information translates to system design optimization, precision in light delivery, and minimization of unintended consequences, such as phototoxicity or photobleaching. In recent years, optogenetics has opened up a new area in deep brain stimulation with light and the method is widely adapted by researchers for the study of the brain circuitries and the dynamics of neurological disorders. A key factor for a successful optogenetic stimulation is delivering an adequate amount of light to the targeted brain objects. The adequate amount of light needed to stimulate each brain object is identified by the tissue optical properties as well as the type of opsin expressed in the tissue, wavelength of the light, and the physical dimensions of the targeted area. Therefore, to implement a precise light delivery system for optogenetics, detailed information about the optical properties of the brain tissue and a mathematical model that incorporates all determining factors is needed to find a good estimation of light distribution in the brain. In general, three measurements are required to obtain the optical properties of any tissue, namely diffuse transmitted light, diffuse reflected light, and transmitted ballistic beam. In this report, these parameters were measured in vitro using intact rat brain slices of 500 μm thickness via a two-integrating spheres optical setup. Then, an inverse adding doubling method was used to extract the optical properties of the tissue from the collected data. These experiments were repeated to cover the whole brain tissue with high spatial resolution for the three different cuts (transverse, sagittal, and coronal) and three different wavelengths (405, 532, and 635 nm) in the visible range of the spectrum. A three-dimensional atlas of the rat brain optical properties was constructed based on the experimental measurements. This database was linked to a Monte Carlo toolbox to simulate light distribution in the tissue for different light source configurations. (Read more in our J. Biomedical Optics paper).

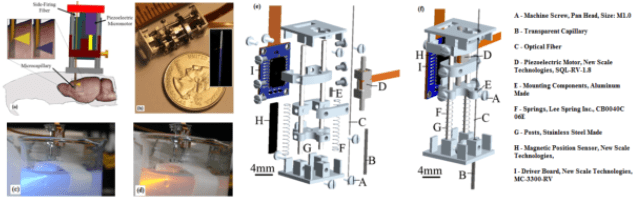
Optical fiber-based probe for optogenetics

With the advent of optogenetics, all optical control and visualization of the activity of specic cell types is possible. We have developed a ber optic based probe to control/visualize neuronal activity deep in the brain of awake behaving animals. In this design a thin multimode optical ber serves as the head of the probe to be inserted into the brain. This fiber is used to deliver stimulation optical pulses and guide a sample of the emission signal back to a detector. The major trade o in the design of such a system is to decrease the size of the ber and intensity of input light to minimize physical damage and to avoid photobleaching/phototoxicity but to keep the S/N reasonably high. Here the excitation light, and the associated emission signal, are frequency modulated. Then the output of the detector is passed through a time-lens which compresses the distributed energy of the emission signal and maximizes the instantaneous S/N. By measuring the statistics of the noise, the structure of the time lens can be designed to achieve the global optimum of S/N. Theoretically, the temporal resolution of the system is only limited by the time lens diraction limit. By adding a second detector, we eliminated the eect of input light fluctuations, imperfection of the optical lters, and back-reection of the excitation light. We have also designed bers and micro mechanical assemblies for distributed delivery and detection of light. (Read more in our IEEE TBME paper).

Side-Firing Fiber: Theory and Fabrication A side-firing fiber is an optical fiber in which the delivery end is angled, and as a result, if designed appropriately, the main lobe of radiation is almost orthogonal to the fiber’s optical axis. Sidefiring fibers have been used for a variety of different biomedical applications including laser therapy, tissue cutting, ablation, etc The side-firing fibers are used to implement the distributed light delivery and detection mechanism in the probe. For this specific application, thin side-firing fibers which are optimized for light delivery and measurements should be designed and fabricated. In this section, we analyze these fibers and introduce a practical approach for the fabrication of the designed fibers.

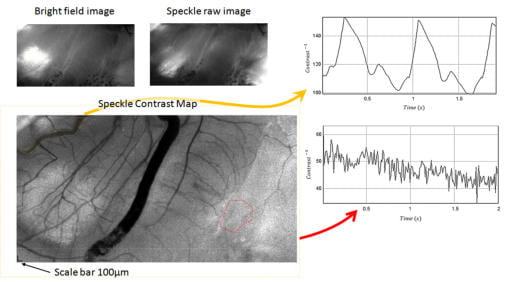
Brain Micro-Angiography via Laser Speckle Contrast Imaging

When an object illuminated by a highly coherent light source, a speckle pattern is produced. This speckle pattern is produced through the mutual interference of sets of wave fronts.If the scattering site has some fluidic motion with moving scatterers such as red blood cells, then a rapid dynamic fluctuation in speckle pattern is observed, due to the constant changes of the scattering site geometry.As the scattering media flow rate increases, the scattering site geometry changes more rapidly, and the amount of time that speckle pattern decorrelated from its previous pattern becomes shorter.
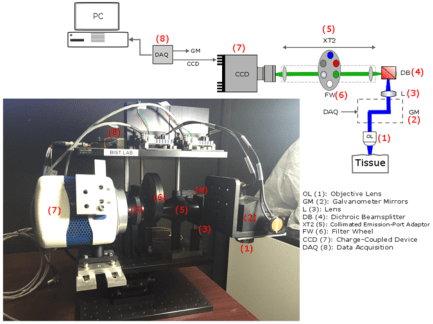
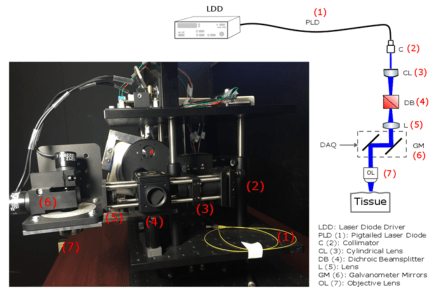
Tissue Analysis of Intermediary scan-lens & tube-lens mechanisms for optical coherence tomography

Combining an optical coherence tomography (OCT) scanner with other techniques such as optogenetic neurostimulation or fluorescence imaging requires integrating auxiliary components into the optical path of the setup. Due to the short scanning distance of most OCT objectives, adding scan and tube lenses in the device is essential to open space between the back-focal-plane of the objective and center of mass of the mirrors in the galvanometer. The effect of the scan and tube lenses on the focal spot size of the scanner using off-the-shelf components are theoretically explored for three different designs in this paper. Two lens mechanisms were implemented and tested in a custom-built OCT scanner to experimentally measure point-spread functions. Based on our analysis, proper form of a four-element semi-Plössl lens provides a superior performance compared with an achromatic doublet when used as a scan/tube lens. The former lens design provides close to diffraction-limited resolution for scan angles up to 6.4degree; however, due to aberrations in an achromatic doublet, the later design offers diffraction-limited resolution confined to 2degree scan angles. (Read more in our recent Applied Optics paper).
Calibration of Digital Optical Phase Conjugation Setup Based on Orthonormal Rectangularl Polynomials
 Digital optical phase conjugation (DOPC) has proven to be a promising technique in deep tissue uorescence imaging. Nonetheless, DOPC optical setups require precise alignment of all optical components to accurately read the wavefront of scattered light in a turbid medium and playback the conjugated beam toward the sample. Minor misalignments and possible imperfections in the arrangement or the structure of the optical components signicantly reduce the performance of the method. In this article, a calibration procedure based on orthogonal rectangular polynomials is introduced to compensate major imperfections including the optical aberration in the wavefront of the reference beam and the substrate curvature of the spatial light modulator without adding extra optical components to the original setup. The proposed algorithm also provides a systematic calibration procedure for the mechanical ne tuning of the DOPC system. It is shown experimentally that the proposed calibration process improves the peak to background ratio by a factor of 8 when focusing light through a highly scattering medium. (Read more in our recent Applied Optics paper).
Digital optical phase conjugation (DOPC) has proven to be a promising technique in deep tissue uorescence imaging. Nonetheless, DOPC optical setups require precise alignment of all optical components to accurately read the wavefront of scattered light in a turbid medium and playback the conjugated beam toward the sample. Minor misalignments and possible imperfections in the arrangement or the structure of the optical components signicantly reduce the performance of the method. In this article, a calibration procedure based on orthogonal rectangular polynomials is introduced to compensate major imperfections including the optical aberration in the wavefront of the reference beam and the substrate curvature of the spatial light modulator without adding extra optical components to the original setup. The proposed algorithm also provides a systematic calibration procedure for the mechanical ne tuning of the DOPC system. It is shown experimentally that the proposed calibration process improves the peak to background ratio by a factor of 8 when focusing light through a highly scattering medium. (Read more in our recent Applied Optics paper).
Fluoresnence Laminar Optical Tomography (FLOT)
Self-Organization in a Parametrically Coupled Logistic Map Network: A Model for Information Processing in Visual Cortex
